Ethanol and its production
FEBRUARY 21, 2022 | @Nokuthula Nchabeleng | BEngHons Chemical Engineering.
What is ethanol?
Ethanol is an organic compound classed under alcohols. It is also known as ethyl alcohol, alcohol, or grain alcohol. Its formula is C2H6O or more commonly C2H5OH where the -OH (hydroxyl group) identifies it as an alcohol. It is a clear, volatile, and colourless liquid with a characteristic odour and high flammability. Ethanol is a product of plant fermentation and can also be produced via ethylene hydration. Ethanol has a range of applications: in the medical field it is a common antiseptic and disinfectant; it is one of the most used organic chemicals in industrial and consumer products and it is also a fuel source.
Production Processes
There are two main types of ethanol – natural ethanol and synthetic ethanol. Chemically, they are indistinguishable; they are rather distinguished by their production method.
Natural Ethanol
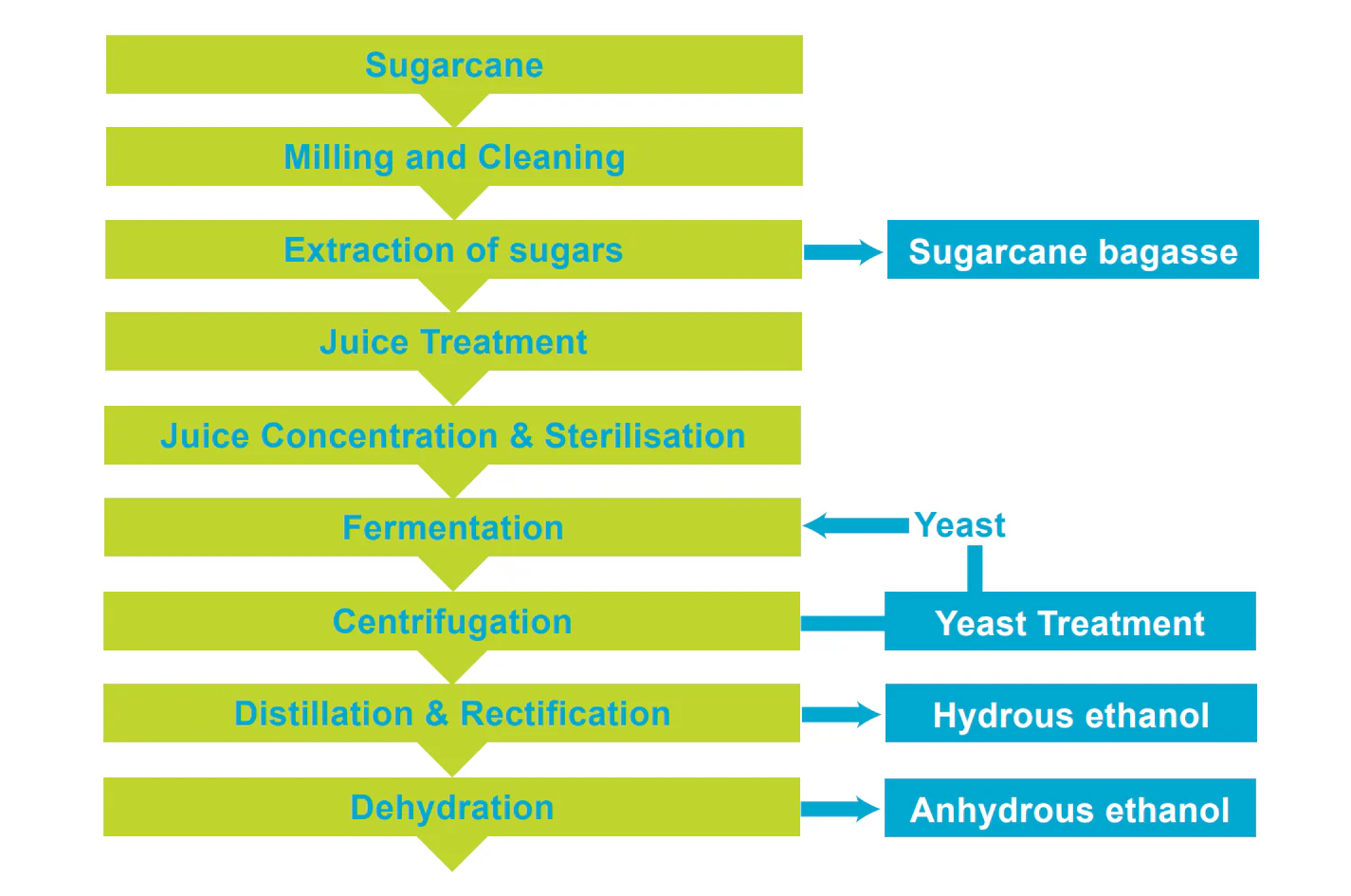
Natural ethanol can be produced from sugar and starchy materials via fermentation with microorganisms such as yeast. When sugarcane is used, a sugar-rich juice is obtained from crushed cane stalks. The juice is then fermented in a tank with yeast. The liquid product formed by the fermentation is then distilled to 92 – 95 % purity. Further separation processes are then employed to obtain the required purity of ethanol. Figure 1.
Process Steps:
- Sugarcane Milling and Cleaning
- Extraction of sugars
- Juice Treatment
- Juice Concentration & Sterilisation
- Fermentation
- Centrifugation
- Distillation & Rectification
- Hydrous ethanol Dehydration to Anhydrous ethanol
Synthetic Ethanol
The first reported synthetic preparation of ethanol was in the 1800s. During this period, a method employing acid-catalysed hydration – similar to that used today in industry – was developed. Today, synthetic ethanol can be produced through either direct or indirect hydration of ethylene.
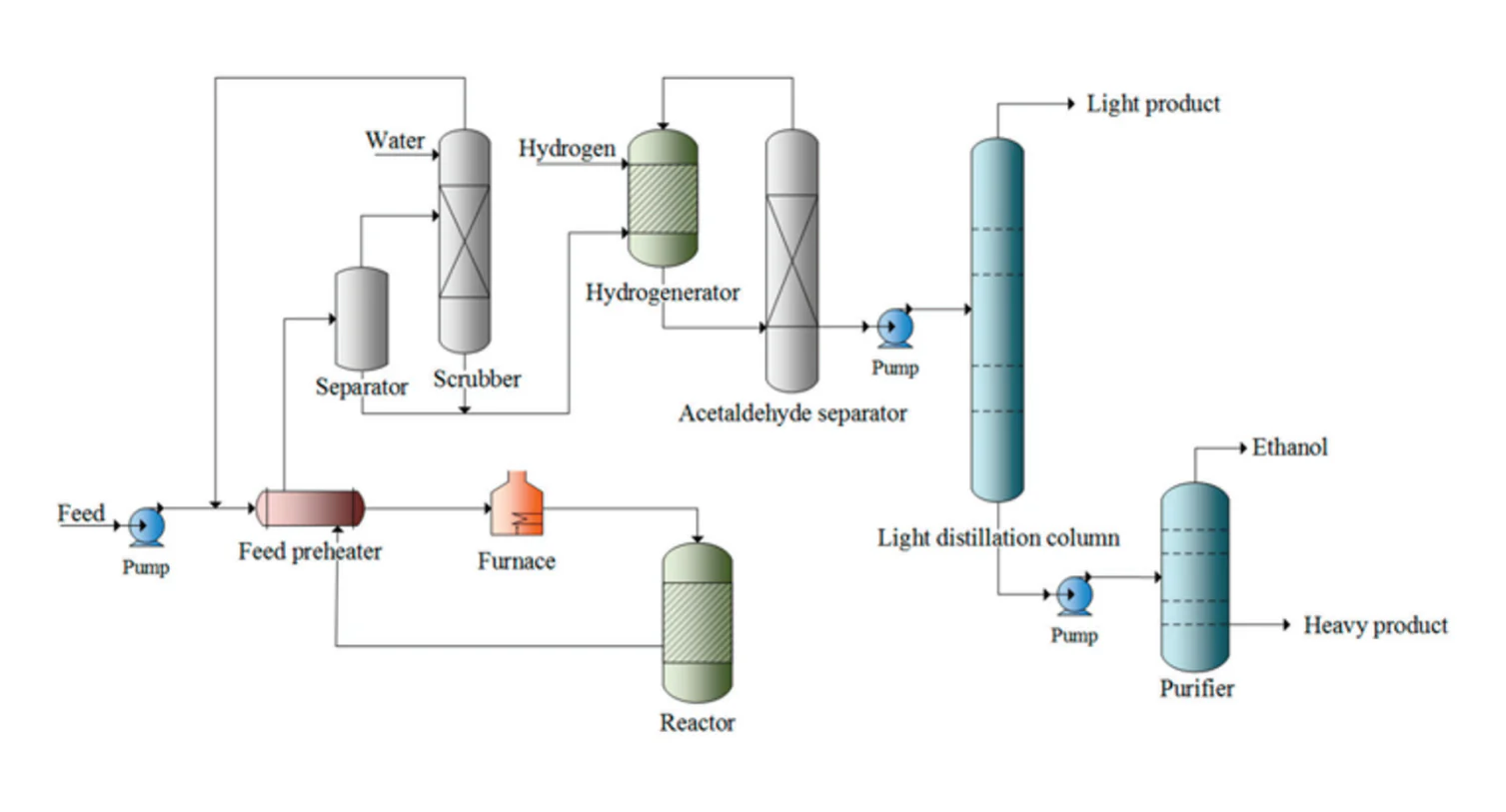
Catalytic direct ethylene hydration comprises of three steps: reaction, recovery, and purification. During the process, a stream of ethylene and steam is preheated and then passed over an acidic catalyst in a packed bed reactor. The remaining reagents are removed from the product mixture in a separator then scrubbed with water to dissolve the ethanol. The two streams formed are then fed to the hydrogenator and purifier where acetaldehyde is converted to ethanol and ethanol is concentrated respectively.
Figure 2 illustrates a simple diagram of the process of direct hydration of ethylene.
Conclusion
iSolvents provides a range of synthetic and natural ethanol products each suited to a variety of applications. Should you require any assistance in your product selection process – we are happy to help.